- News & Blog
- How The Healthcare Industry is Responding to Testing Needs
How The Healthcare Industry is Responding to Testing Needs
The healthcare industry has always been at the forefront of addressing global health challenges. However, the recent pandemic has underscored the critical importance of rapid, accurate, and accessible testing. The response to these testing needs has been multifaceted, involving technological advancements, innovative logistical solutions, and comprehensive regulatory compliance. At BioTouch Global, we play a pivotal role in supporting these efforts through our specialized logistics and kitting solutions. Here’s how the healthcare industry is rising to meet the increasing demand for testing.
1. Technological Advancements in Testing
Innovative Diagnostic Technologies
The development of new diagnostic technologies has been accelerated to meet the urgent demand for testing. Innovations such as rapid antigen tests, molecular diagnostics, and next-generation sequencing have significantly enhanced the ability to detect diseases quickly and accurately. These technologies are crucial in managing not only infectious diseases but also chronic conditions and genetic disorders.
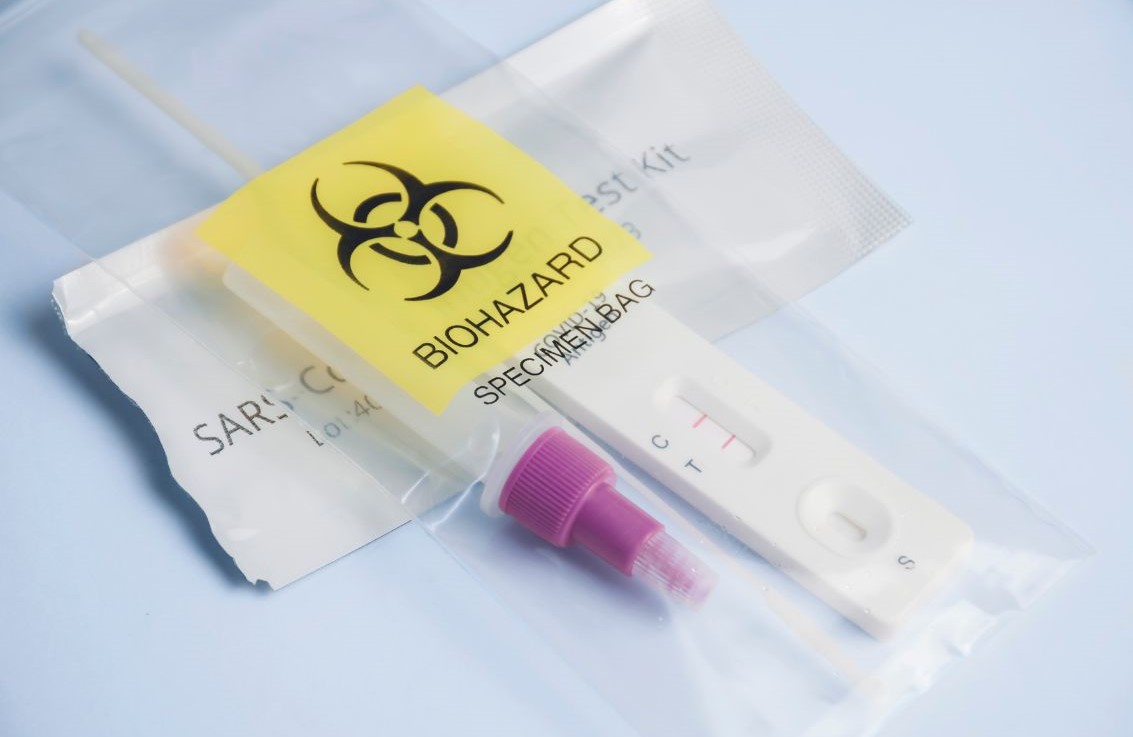
Point-of-Care Testing
Point-of-care testing (POCT) has gained prominence due to its convenience and rapid results. POCT allows healthcare providers to perform tests at or near the site of patient care, leading to quicker decision-making and improved patient outcomes. The use of portable devices and easy-to-use kits makes POCT a vital tool in various healthcare settings, from hospitals to remote clinics.
2. Expanding Testing Infrastructure
Increased Testing Capacity
To meet the surge in testing demand, laboratories and healthcare facilities have expanded their capacities. This includes the establishment of new testing centers, upgrading existing facilities, and enhancing laboratory capabilities to handle higher volumes of tests. Such expansions are essential to reduce turnaround times and ensure timely diagnosis and treatment.
Mobile Testing Units
Mobile testing units have become an innovative solution to reach underserved and remote populations. These units are equipped with the necessary tools and personnel to conduct on-site testing, thereby increasing accessibility and ensuring that more people can be tested regardless of their location.
3. Enhanced Logistics and Supply Chain Management
Efficient Specimen Collection and Transport
The logistics of specimen collection and transport have seen significant improvements. Efficient supply chain management ensures that specimens are collected, transported, and processed without delay. At BioTouch Global, we provide advanced logistics solutions, including specialized kitting and direct-to-patient delivery services, to streamline these processes and maintain the integrity of samples.
Temperature-Controlled Solutions
Certain tests require specimens to be maintained at specific temperatures to ensure accuracy. Our temperature-controlled solutions, such as insulated shippers and gel packs, are designed to meet these requirements. This ensures that specimens remain viable from the point of collection to analysis, regardless of external conditions.
4. Regulatory Compliance and Quality Assurance
Adhering to Regulatory Standards
Meeting regulatory standards is critical to ensure the safety and efficacy of testing processes. The healthcare industry must comply with various regulations, such as the FDA's Good Manufacturing Practices (GMP) in the U.S. and the EU's In Vitro Diagnostic Regulation (IVDR). At BioTouch Global, our facilities are ISO 13485 certified, reflecting our commitment to maintaining the highest quality and compliance standards.
Continuous Improvement
The dynamic nature of healthcare demands continuous improvement in testing protocols and logistics. Regular audits, staff training, and process optimization are essential to adapt to evolving regulatory requirements and industry best practices. This proactive approach ensures that testing remains reliable and effective.
5. Collaborations and Partnerships
Public-Private Partnerships
Collaborations between public health authorities and private companies have been instrumental in scaling up testing capabilities. These partnerships facilitate resource sharing, technological innovation, and streamlined operations. By working together, stakeholders can address testing needs more efficiently and effectively.
Global Coordination
Global coordination is vital to managing testing on an international scale. Organizations such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) provide guidelines and support to standardize testing protocols worldwide. Such coordination helps in the timely identification and control of outbreaks.
Conclusion
The healthcare industry’s response to the growing testing needs has been comprehensive and dynamic. Through technological advancements, expanded infrastructure, enhanced logistics, strict regulatory compliance, and strategic collaborations, the industry is better equipped to handle current and future challenges. At BioTouch Global, we are proud to contribute to these efforts by providing the logistics and kitting solutions that underpin successful testing operations.